DOSING AND TITRATION WITH 10-MG FIRDAPSE TABLETS
For patients ≥6 years of age and weighing ≥45 kg2*
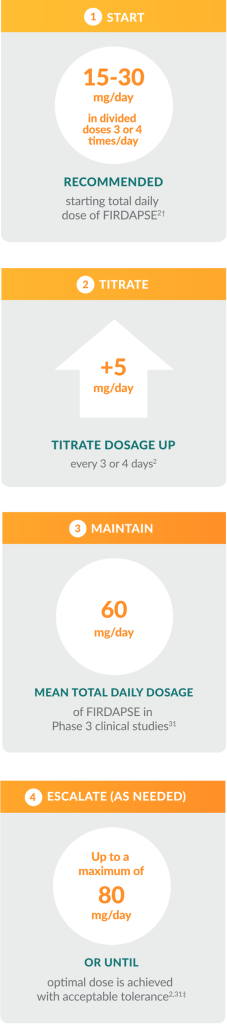
Help your patients reach their optimal therapeutic dose
Titration is an important step for new patients who are just getting started on FIRDAPSE and have not taken amifampridine before. By guiding your patients through the titration process, you can help:
- Determine their optimal therapeutic dose of FIRDAPSE
- Maximize neuromuscular benefit
- Minimize issues with tolerability, including paresthesia
ADDITIONAL DOSING INFORMATION
- FIRDAPSE tablets can be taken with or without food2
- Scored tablets can be split with a pill splitter as needed2
- NOTE: If you have dosing adjustments <5 mg or have patients who have trouble swallowing a tablet, a suspension can be prepared. Refer to the Medication Guide for instructions on suspension preparation.2
- If a dose is missed, patients should not take double or extra doses2
- Patients newly treated with amifampridine should be advised that based on clinical trial observations, they may experience transient paresthesia, but this side effect is expected to diminish with continued treatment2,28
- In patients with renal or hepatic impairment and individuals known to be poor metabolizers of N-acetyltransferase 2 (NAT2), the recommended starting dose of FIRDAPSE is the lowest recommended initial daily dosage taken orally in divided doses. This dose should continue to be titrated to clinical effect and tolerability.2§
HOW FIRDAPSE IS SUPPLIED
FIRDAPSE is dispensed in both bottles and child-resistant blister packs, making it easy for you and your patients to choose the best option for them.
Bottles2
- Containing 60 or 240 tablets
Blister packs2
- Individual pack containing 10 tablets
- Carton containing 12 packs (120 tablets total)

Do you have a patient taking FIRDAPSE who is pregnant?